21+ Calculate The Molar Mass For Mg Clo4 2
For the reaction shown calculate how many moles of each product form when the given amount of each reactant completely reacts. The molar mass of KNO3 is 10111 gmol.

Compounds 1 Types Of Compounds 2 Formula Writing 3 Formula Naming 4 Empirical Formulas 5 Molecular Formulas 6 Types Of Chemical Reactions 7 Balancing Ppt Download
Enter the email address you signed up with and well email you a reset link.

. Lecture notes in General and Inorganic Chemistry provides an introduction to the chemistry of inorganic molecules. A 152 1027 sodium ions B 476 1020 sodium ions C 210 1021 sodium ions D 105 1021 sodium ions E 952 1020 sodium ions. A 219 cm3 B 0456 cm3 C 285 cm3 D 124 cm3 E 605 cm3 Iron has a density of 786 gcm3.
Titrimetric methods include powerful group of quantitative procedures that are based on measuring the amount of reagent consumed by the analyte. Integrative and Advanced Exercises 57. Calculate the molar mass of Ca3PO42.
Eq If K eq 1x10-6. 100 g C14H18N2O5 c. Express the number of moles of Al S and O atoms numerically separated by commas Calculate the number of moles of magnesium chlorine and oxygen atoms in 560 moles of magnesium.
Transcript 10 mol F2 440 g CO2 40 g H2 146 g SF6 34. The volume occupied by 5585 g of iron is A 0141 cm3 B 711 cm3 C 28 cm3 D 439 cm3 E None of the. A 8705 gmol B 21521 gmol C 31018 gmol D 27921 gmol E 24618 gmol.
There are 3 mol S8 produced per 8 mol SO2 that react. If the molar mass is 144 gmol what is the molecular formula. Calculate the mass of a cylinder of stainless steel 1d 775 gcm32 with a height of 1835 cm and a radius of 188 cm.
The molar mass of Na2SO3 is 12605 gmol. 15-10 eing If Keq is 1x10¹ calculate AGCO If K is 1 calculate AG. Aq NiClO42aq 2 Ags Ag.
3 NO2g H2Ol 2 HNO3aq NOg A 281 moles NO B 253 moles NO C 844 moles NO D 550 moles NO E 183 moles NO Consider the following reaction. 15999 g 294305 gmol mol O 1 mol C14 H18 N 2 O 5 340 102 mol C14H18N2O5 2943 g C14 H18 N 2 O 5 2943 g 459 g C14H18N2O5 mol 1g 1 mol 602 10. These methods include i.
C3H8g5O2g3CO2g4H2Og a 42 mol C3H8 b 42 mol C3H8 c 00567 mol C3H8 d 00567 mol C3H8 e 42 mol O2 f 42 mol O2 g 00567 mol O2 h 00567. Calculate the volume occupied by 250 g of lead. In addition the estimated detonation velocity of molecular ferroelectrics can be tuned from 669 021 to 779 025 km s1 by switching the polarization state.
Study with Quizlet and memorize flashcards containing terms like chapter 1 que 32 The density of lead is 114 gcm3 at 25C. Calculate the molar mass for MgClO42. These methods include i.
Based on this value A. The periodic table can be used to calculate the molar mass of any substance. Study with Quizlet and memorize flashcards containing terms like Calculate the number of moles of aluminum sulfur and oxygen atoms in 300 moles of aluminum sulfate Al2SO43.
Mass of sample 220 g Mass of NiOH2 295 mg known. Assume that there is more than enough of the other reactant. Predissociation Dynamics of Br 2 in the 2 Π 12 c 5d.
770 g SO21 mol SO264064 g SO23 mol S88 mol SO2256528 g S81 mol S8116 g S8 Next calculate the amount of S8 that can be produced from 770 g H2S and excess SO2. 50 mg e. 0 g Rydberg States by Velocity Map Imaging Study Shoma Hoshino Kento Ishii.
S8has a molar mass of 832066256528 gmol. FE Reference Handbook 1001. How would the concentration change if a 10 L flask of 10 M NaCl were left uncapped on a laboratory bench for several days.
14 mol C H 12011 g 10079 g 14007 g 18 mol H 2 mol N mol C mol H mol N 5 mol O b. Write a balanced equation to show the reaction of sulfurous acid. The emphasis is on basic principles of atomic and molecular structure thermodynamics chemical kinetics and catalysis properties of solutions acid-base equilibria hydrolysis and buffer solutions and coordination compounds.
Since the ratio is 11 the number of moles of Na2S is equal to the number of moles of CdNO32. Titrimetric methods include powerful group of quantitative procedures that are based on measuring the amount of reagent consumed by the analyte. Calculate the molar mass for MgClO42.
The pH of human blood is controlled to be within the range of 735 to 745 primarily by the carbonic acid-bicarbonate buffer system. These methods include i. 4 KNO3s 2 K2Os 2 N2g 5 O2g.
4NH3 5O2 4NO 6H2O. Which of the following equations will give the correct formula mass of the compound FeClO42. 156 mol d.
Formula and molecular masses are calculated using the chemical _____ of the relevant compound and the atomic masses obtained from the _____ table. How many sodium ions are contained in 996 mg of Na2SO3. The molar solubility of CaOH2 was experimentally determined to be 0022 M.
ML of 150 10-4 M hydrochloric acid is added to 135 mL of 175 10-4 M MgOH2. CO 2 g 1 H 2 Ol 8 H 2 CO 3 aq H 2 CO 3 aq 1 H 2 Ol 8 H 3 O 1 aq 1 HCO 3 2 aq This chapter describes polyfunctional acid and base systems including buffer solutions. A Mg 24305 Cl35453 O159994 243053545321599944222321 gmol.
The volume or mass of the reagent needed to react completely with a fixed quantity of the analyte is obtained from which the amount of analyte is determined. Calculate the molar mass for AlC2H3O23. The volume or mass of the reagent needed to react completely with a fixed quantity of the analyte is obtained from which the amount of analyte is determined.
To determine the mass of Na2s multiply the number of moles by the mass of one mole. What is the pH of a solution formed by mixing 1350 mL of 00100 M HCl with 800 mL of 00450 M. Mass of one mole 2 23 321 781 Mass 000025 781 0019525 grams This is 19525 mg.
H2S has a molar mass of 21007932066340818 gmol. 0 g and 2 Π 32 c 6d. The volume or mass of the reagent needed to react completely with a fixed quantity of the analyte is obtained from which the amount of analyte is determined.
Study with Quizlet and memorize flashcards containing terms like According to the following balanced reaction how many moles of NO are formed from 844 moles of NO2 if there is plenty of water present. How many grams of acetic acid are present in 100 L of this vinegar. Click to see the answer Q.
A 22321 gmol B 12376 gmol C 11952 gmol D 24752 gmol E 7576 gmol. Calculate the mass of a block of iron 1d 786 gcm32 with dimensions of 528 cm 674 cm 373 cm. C8H16O2 Which of the following stoichiometric factors would be used to convert from moles of NH3 to moles of H2O for the reaction.
Molar mass of Ni 5869 gmol Molar question_answer Q. Calculate the molar mass of Ca3PO42. Titrimetric methods include powerful group of quantitative procedures that are based on measuring the amount of reagent consumed by the analyte.
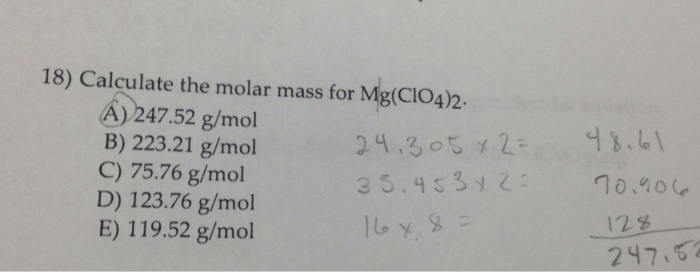
Solved Calculate The Molar Mass For Mg Clo 4 2 A 247 52 Chegg Com

Three Giant Lanthanide Clusters Ln37 Ln Gd Tb And Eu Featuring A Double Cage Structure Inorganic Chemistry

Identify The Polyatomic Ions In The Following Compounds And Calculate The Molar Mass Of The Compound A Mgco3 B Na2so4

R Hill The Mathematical Theory Of Plasticity Pdf Plasticity Physics Yield Engineering
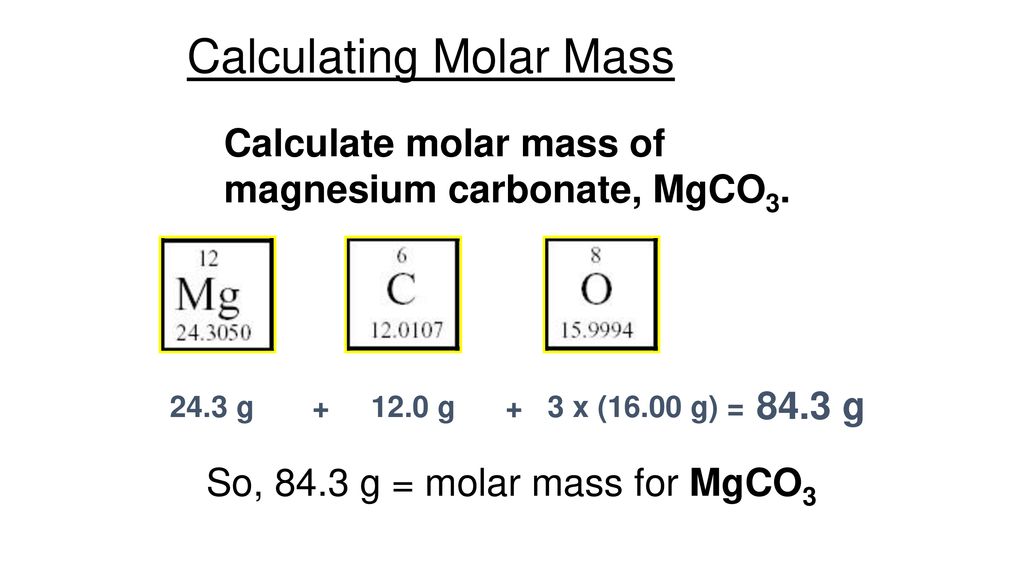
The Mass Of A Mole Of An Element Ppt Download

How To Find The Number Of Atoms In Mg Clo4 2 Magnesium Perchlorate Youtube

Chemical Measurements Unit 1 Stoichiometry Chapter 10 The Mole Ppt Download

The Mole As Ppt Download
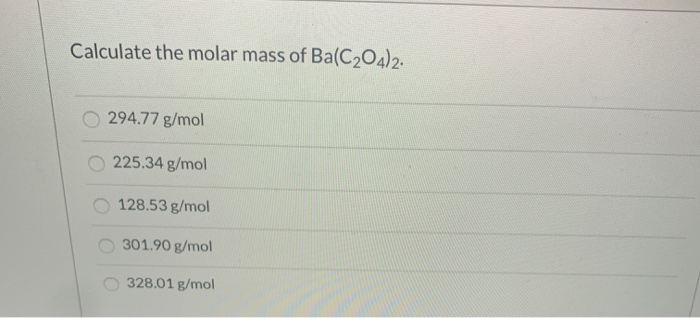
Solved Calculate The Molar Mass Of Ba C2o4 2 294 77 G Mol Chegg Com

Pdf Caqs Ebook Alex Milliron Academia Edu

Moles And Formula Mass Ppt Download

Passes 4 Oxygens Ate Ending Ppt Download

Kupdf Net Chemistry Of Elements 2nd Ed N N Greenwood Amp A Earnshaw 2

Molar Mass Molecular Weight Of Mg No3 2 Magnesium Nitrate Youtube

Calculate The Molecular Mass Of The Following Compounds 1 Naoh 2 Mg No3 2 3 Caco3 4 Mgco3 Brainly In

Calculating Molar Mass Of Mg Oh 2 Youtube

Stoichiometry And The Mole Ppt Video Online Download